HMG Benefits |Side effect | DOSAGE |Reviews
HMG is an indication for use in combination with chorionic gonadoopin, is used for primary or secondary amenorrhea caused by insufficient gonadotropin secretion, infertility caused by anovulatory rare menstruation.
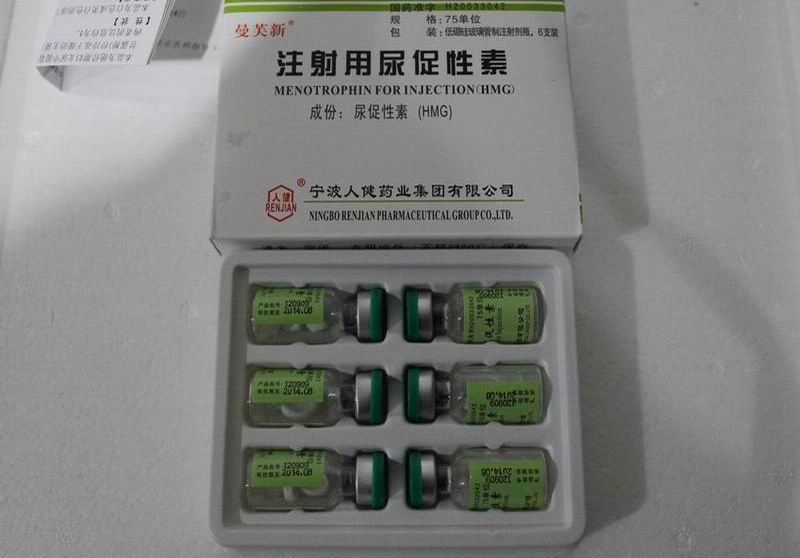
| English name | Menotropins for Injection | |
| Specifications | 75 units | |
| Packaging | 75 units | |
| Dosage | this product is soluble in 1-2 mL of sodium chloride injection, intramuscular injection. Start (or from the 5th day of the cycle) once 75 units, once a day. The dose was adjusted after seven days based on the patient’s estrogen levels and follicular development. If the ovary does not respond, increase 75 units every seven days from the second week, but the maximum dose does not exceed 225 units. Up to 10,000 units of chorionic gonadotropin (HCG) were used after the follicles matured, and an intramuscular injection induced ovulation. If the ovary does not respond after 3 weeks of injection, the drug is stopped. | |
| Dosage form | injection | |
| Character | this product is a white or off-white lyophilized cake or powder. This product is dissolved in water. | |
| Validity period | 18 months | |
| Country/Region-made | Production Enterprise Livzon Group Livzon Pharmaceutical Factory |
Indications
This product is combined with chorionic gonadotropin for primary or secondary amenorrhea caused by insufficient secretion of gonadotropin, infertility caused by ovulation-free thin menstruation.
Adverse reactions
1. Mainly ovarian hyperstimulation syndrome, manifested as lower abdominal discomfort or bloating, abdominal pain, nausea, vomiting, ovarian enlargement. Severe can cause chest tightness, shortness of breath, decreased urine output, pleural effusion, ascites, and even rupture of follicular cysts.
2. If the ovary suddenly increases after stimulation, the development of multiple follicles may have ovarian torsion or rupture of the ovarian cyst, or even intra-abdominal hemorrhage. It is generally possible that the symptoms are aggravated 3 to 10 days after the injection of HCG to promote ovulation.
3. The use of this product can often increase the risk of arterial embolism.
4. There are still multiple pregnancies and premature births.
Contraindications
Abnormal vaginal bleeding, uterine fibroids, ovarian cysts, ovarian enlargement, adrenal insufficiency, thyroid insufficiency, and primary ovarian failure in patients with unknown causes.
Note 1. It should be administered under the guidance of an experienced gynecological endocrinologist. Note during medication
Intentional monitoring
(1) Thorough pelvic examination to understand the size of the ovaries, especially after the estrogen concentration begins to rise, check daily until at least 2 weeks after the addition of velvet.
(2) Measuring basal body temperature every day helps to understand ovarian ovulation.
(3) Determination of estrogen excretion, one week after using this product, daily urine or blood to measure estrogen. The use of velvetin is only started 24 hours after the peak of estrogen. If the estrogen value is too high, it is not appropriate to give a large amount of HCG, so as not to cause excessive stimulation of the ovary.
(4) Cervical mucus examination can help to understand the degree of follicle maturation or whether there is ovulation.
(5) Check β-HCG for early pregnancy.
(6) For patients with high LH values, such as polycystic ovary syndrome, gonadotropins containing only FSH 75U should be used.
2. Athletes use with caution.
3. If severe ovarian hyperstimulation syndrome occurs, discontinue the drug immediately.
4. Asthma, heart disease, epilepsy, renal insufficiency, pituitary tumor or hypertrophy, thyroid or adrenal insufficiency in patients with caution.
5. The pregnancy after the use of this product combined with chorionic gonadotropin has a report of stillbirth and congenital malformation, but it has not been directly related to this product.
6. In the treatment of this product, the follicles are more than 20mm in diameter when the follicles mature, and the estrogen content is up to 100-150μg in 24 hours. The gonadotropin can be injected. If the above indicators are exceeded, the symptoms of ovarian hyperstimulation should be stopped. medicine.
7. Waste pharmaceutical packaging should not be discarded at will.
Drug Interactions
Drug interactions may occur if used in conjunction with other drugs. Consult your physician or pharmacist for details.
Storage
sealed